
For decades, hormone replacement therapy (HRT) has been a well-known aid to symptoms prevalent in postmenopausal women. Among these symptoms, it is mostly used to treat osteoporosis. Two new drugs, alendronate sodium and raloxifene hydrochloride were recently approved by the Food and Drug Administration (FDA) for the prevention of osteoporosis as an alternative to HRT. As data is limited on these drugs, a study was done to learn more of the efficacy of each drug, and their short term side effects to prove whether it is more or less effective than HRT for postmenopausal women. The risks on hip fracture, coronary heart disease (CHD), breast cancer and life expectancy were also compared for the three treatment methods.
It is not unusual for postmenopausal women to have concerns about the life long consequences of estrogen deficiency. Widely used HRT, estrogen combined with progestin, averts accelerated bone loss that happens after menopause and may even decrease the risk of developing CHD. It may however increase the risk of breast and endometrial cancers. The new therapies, alendronate sodium and raloxifene hydrochloride, have been studied as new sources for estrogen replacement and have established to decrease the risk of verbal fractures but without the same cancer risks caused by HRT.
Both however, also lack some of the advantages associated with HRT. Raloxifene, a nonsteroidal compound with mixed estrogenic and antiestrogenic properties, does not increase the risk of endometrial cancer, may decrease the risk of breast cancer and can lower serum total cholesterol levels. Alendronate, a bisphoshonate, may inhibit bone resorption and has no known impact on CHD or either cancers associated with HRT.
For over 30 years, postmenopausal women taking HRT have experienced a decrease in fracture rates, the incidence of CHD and an increase in survival rates. Because they are new, raloxifene and alendronate therapy could only go through limited measures of clinical efficacy. Each will require decades of follow-up studies to determine a more accountable measure of impact on hip fracture and CHD.
The study was done on healthy, white 50 year old postmenopausal women who were receiving one of the three treatments for estrogen deficiency.
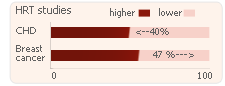
Effects of HRT
Studies suggest that HRT:
- Lowers the risk of developing CHD by 40%
- Increases the risk of breast cancer by 47%
- Shows no impact (when used with progestin) on endometrial cancer
Effects of Raloxifene Therapy
Long term impact of raloxifene therapy on breast cancer cannot yet be established. During the three years of randomized studies of 10,385 postmenopausal women taking raloxifene there was an enhanced chance of .38% for breast cancer. This test however assumes that raloxifene therapy only lowers the risk of breast cancer during the first five years of treatment, rather than stopping it completely.
Impact of Life Expectancy
HRT increased life expectancy among postmenopausal women more than raloxifene and alendronate therapy. It is wise to note that life expectancy gains depended on the individual woman's risk profile thus no treatment was proved better or worse for life expectancy. However, women at a high risk for CHD should take HRT while women at high risk for breast cancer would gain from taking raloxifene.
Impact on Hip Fracture
All three therapies showed similar efficacies in preventing hip fractures.
As HRT may increase the risk of breast cancer, a fear more prevalent the CHD or hip fracture after menopause, a woman must decide whether the gain in life expectancy is worth the risk of breast cancer. Still, more information is needed on both raloxifene and alendronate therapy. As it stands today, women have more choices on estrogen replacement other than HRT, but the newly released approvals of the FDA fail to provide sufficient information on their efficacy.